This article originally appeared in the March/April 2023 issue of Glass Worldwide Magazine. Reprinted with permission.
In various borosilicate glassmaking, anhydrous borates (boron species without crystal water), such as Dehybor®, bring multifaceted benefits of value-in-use (VIU)[1]. One of the most intuitive benefits is reducing glass manufacturers’ energy use. Compared to boron species with crystal water, anhydrous borates thermodynamically require less energy to melt.
However, glass producers in a variety of production environments still find it a challenge to quantify the energy efficiency gains of using anhydrous borates. Factors such as glass compositions, raw materials, fuel type, furnace age, and furnace settings may affect how much energy reduction could be realized in glass factories. Traditional measurement methods, such as plant trials, are difficult and costly to execute. They require stable conditions to account for variables and inevitably cause disruption to production before and after the trial. Both raw material suppliers and glassmakers need a faster, more precise assessment for anhydrous borates—one that can consider complication of using increased share of electric energy and can convert energy reduction into total carbon emission reduction.
With this new method, glassmakers can measure how they are contributing to a decarbonized glass industry.
Two step changes to improve assessment methods
Traditionally, the glass industry used an entropic desktop calculation of various state of boron species to assess the energy reduction of using anhydrous borates. While this academic approach provides the “book ends” assessment, it is too general to consider different glass compositions and chemical reactions among different glass batches.
Therefore, the first step-change improvement is using time-dependent batch energy measurement system developed and performed by CelSian, or high temperature drop calorimetry (HTDC) setup. HTDC is designed to measure energy intake in kilowatt within a confined electric furnace system during batch-free melting trials. In this real lab setup, researchers can measure how much energy different glass batches must consume to reach an equilibrium melting state. On one hand, using an electric system enables precise energy measurement. But on the other hand, this approach brings the disadvantage of “dried” industrial flue gas conditions which fails to consider the impact from fossil fuel combustion.
Consequently, the second step-change improvement introduced computer modeling, or energy balance modelling (EBM), developed and performed by CelSian. This modelling enables glass producers or researchers to input results from HTDC and to incorporate operational parameters (eg, furnace setup, size, pull, temperature profile, and percentage of electric booster).
As a result, the output is not only able to simulate industrial flue gas conditions but also to provide net carbon emission reduction from using anhydrate boron species. This new approach of using lab-based measurement plus computer modeling only takes hours to run and gives more realistic and tailored results to individual glassmaker.
 Figure 1: HTDC measurement tools[2]
Figure 1: HTDC measurement tools[2]
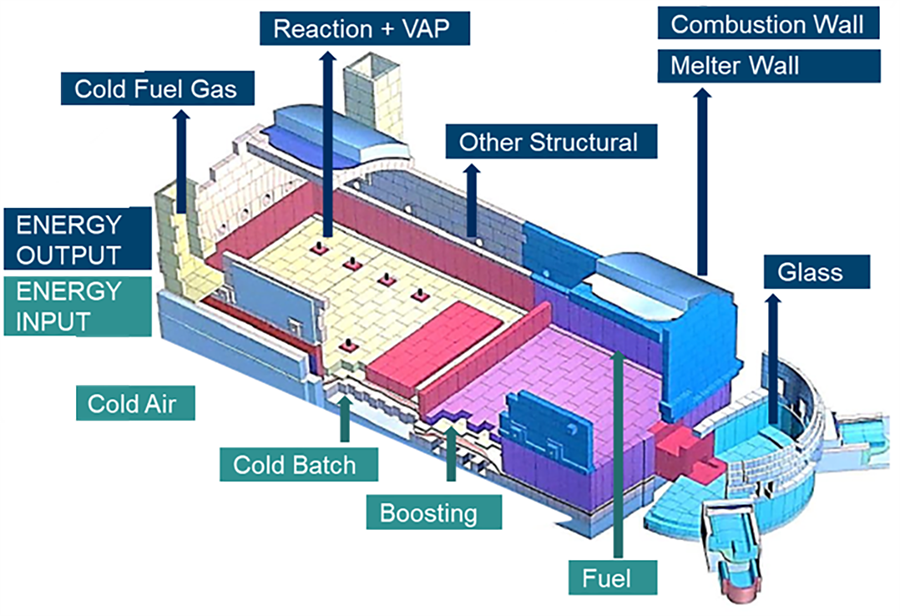 Figure 2: EBM model schematic[2]
Figure 2: EBM model schematic[2]
Three studied glass applications as examples
So far, U.S. Borax studied three typical glass applications using HTDC and EBM: Insulation glass wool, Type I pharmaceutical glass, and TFT display glass. Table 1 outlines some of the parameters chosen in the study. Figures 3, 4, and 5 show results based on general compositions and batch compositions of three glass types.
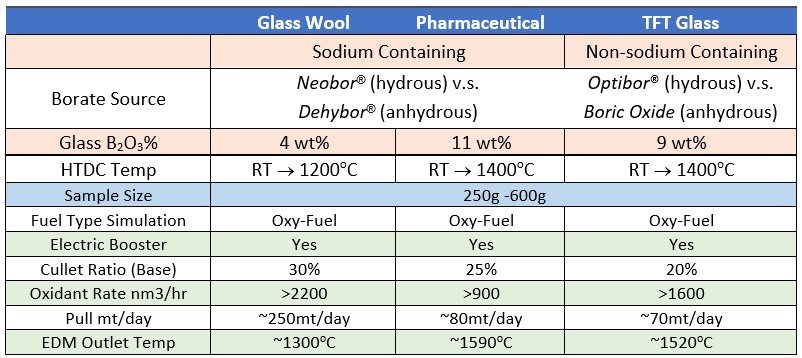 Table 1: B2O3% and temperature used of three applications in HTDC
Table 1: B2O3% and temperature used of three applications in HTDC
From Figure 3: Using anhydrous product achieved different, yet remarkable, energy savings across all three glass applications. B2O3 wt% chosen are meant for industrial average as central case. Note: The reduction percentage increased when a higher B2O3% is present in glass.
Figure 4 shows the results of EBM results. In EBM, the energy balance reviewed energy to structural loss, energy to flue gases and energy to glass. The EBM results revealed that energy savings is attributed to less reactive gases in the batch and reduced energy loss by flue gases leaving the furnace. Compared to HTDC, the energy reduction percentages are reduced for two primary reasons: (1) simulation of internal cullet ratio in EBM and (2) energy loss of furnaces. In our studies, internal cullet shares the same composition with final products. Its ratios were also changed during EBM exercise to understand the sensitivity.
Consequently, Table 2 shows CO2 reduction percentage results from using anhydrous borates. This percentage was slightly diluted when compared to energy reduction. This is because of: (1) The use of electric energy in the simulation (2) the use of dolomite and limestone (carbonate containing) for a calcium source in the batch.
Finally, our study also used EBM to understand the capacity benefit of using anhydrous borates. When the total energy input is set to the same amount, incremental furnace pull can be quantified with the use of anhydrous borates. However, this approach does not consider that defect rate might increase. Depending on glass applications and individual operational constraints, the utility of this approach may differ. For some that could realize the capacity benefit of using anhydrous borate, this approach yields a pronounced unit reduction of specific energy consumption and specific CO2 emission. This is an alternative route for consideration, made possible by EBM. The impact of a pull increase on glass quality and furnace lifetime can be further assessed by detailed glass furnace simulation.
It is important to note that all these results are based on a series of furnace settings and assumptions designed for an industrial-average base case. The HTDC and EBM approach enables fast and specific settings changes to suit different environments. Collaborating with CelSian, U.S. Borax has gathered a series dataset of HTDC ready for fast EBM modeling and will continue work closely with interested glassmakers to understand specific benefits of using anhydrous borates.
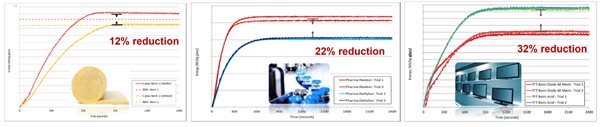 Figure 3: HTDC results for (a) Glass wool (b) Type 1 pharmaceutical glass (c) TFT glass
Figure 3: HTDC results for (a) Glass wool (b) Type 1 pharmaceutical glass (c) TFT glass
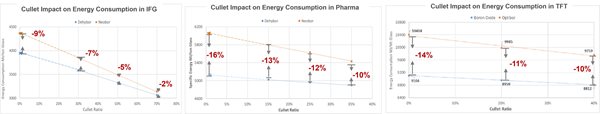 Figure 4: EDM results as function of internal cullet ratio for (a) Glass wool (b) Type 1 pharmaceutical glass (c) TFT glass
Figure 4: EDM results as function of internal cullet ratio for (a) Glass wool (b) Type 1 pharmaceutical glass (c) TFT glass
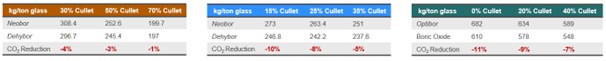 Table 2: CO2 reduction as a function of cullet ratio for (a) Glass wool (b) Type 1 pharmaceutical glass (c) TFT glass
Table 2: CO2 reduction as a function of cullet ratio for (a) Glass wool (b) Type 1 pharmaceutical glass (c) TFT glass
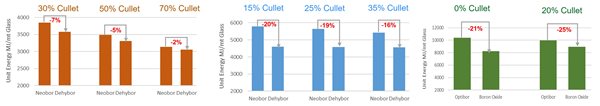 Figure 5: Unit energy reduction due to increased pull rate for (a) Glass wool (b) Type 1 pharmaceutical glass (c) TFT glass
Figure 5: Unit energy reduction due to increased pull rate for (a) Glass wool (b) Type 1 pharmaceutical glass (c) TFT glass
Glassmakers scope 3 emissions
The glass industry is increasingly concerned with not only reducing carbon footprint during glassmaking (Scope 1), but also reducing upstream Scope 3 emissions related to raw materials. Unlike carbonate raw materials such as limestone (CaCO3), dolomite (CaMg(CO3)2), and soda ash (Na2CO3) which produce CO2 emissions when heated in glass furnaces, refined borates do not generate carbon emissions in glassmaking. However, the carbon footprint of borate production and transportation does contribute to total glass CO2 emissions. Therefore, it is critical for glassmakers to understand the environmental impact of the borates used in their process. Understanding this impact, empowers glassmakers to make an informed decision on what type of borate to use and from which supplier.
The carbon footprint of borates depends on the whole production process including supplied raw materials, type of borate mineral used, mining operations, refining process, packaging, and the input energy used for production process. Therefore, not all borates are equal when it comes to environmental impact. Life cycle assessment (LCA) provides the best framework to assess and determine the environmental impacts of borate products. U.S. Borax is committed to evaluate the environmental impact of its products by partnering with certified ESG consultants to create cradle-to-gate LCA data that includes upstream Scope 3, Scope 1, and Scope 2 emissions.
Analyzing the current environmental impact is the first step in our journey toward reducing CO2 emissions and meeting sustainability goals. Glassmakers can include LCA data from suppliers in their own glassmaking LCA study. Figure 6 shows the carbon footprint of borax pentahydrate produced by U.S. Borax (Neobor) compared with two alternative products based on cradle-to-gate LCA studies[3-4]. In taking reference from the results of the independent studies as shown below, it would appear Neobor production has a significantly lower CO2 emissions rate compared to benchmarks, meaning glassmakers can reduce their product carbon footprint by selecting raw material with the lowest environmental impact.
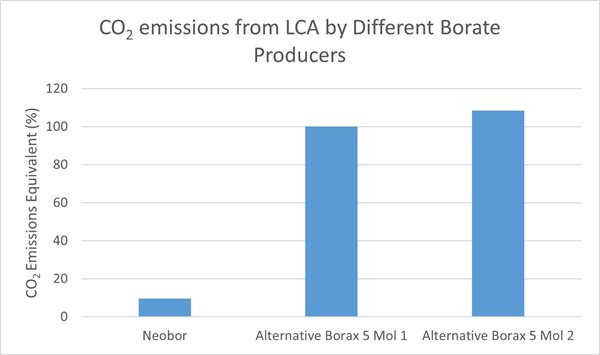 Figure 6: CO2 emissions from LCA for borax pentahydrate by different borate producers; functional unit is Kg of CO2 per metric ton of borate [3-4]
Figure 6: CO2 emissions from LCA for borax pentahydrate by different borate producers; functional unit is Kg of CO2 per metric ton of borate [3-4]
Beyond CO2 emissions in production, raw material transportation contributes to upstream Scope 3 emissions for glassmakers. Transportation emissions depend on factors such as distance between suppliers and customers, transportation mode, and the type of fuel used. However, the type of borate—anhydrous vs hydrous—can also significantly impact transportation emissions. Using anhydrous borates such as Dehybor and boric oxide for applications spanning insulation glass wool, pharmaceutical glass, and TFT glass lowers carbon emissions in transportation compared to their hydrous counterparts (Neobor and Optibor). Anhydrous borates contain a higher of B2O3 concentration than hydrous borates, meaning less product needs to be transported. That lowers both carbon emissions and transportation costs. Figure 7 compares the boric oxide content of anhydrous vs. hydrous borates. For example, in TFT glass application, 1 mt of Optibor should be transported to the glassmaker in order to obtain 0.57 mt of B2O3. That requires transporting 75% more product by weight, increasing the glassmaker’s carbon footprint.
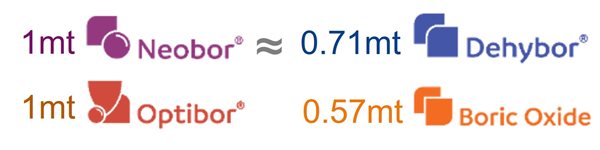 Figure 7: Comparison of hydrated and anhydrous borates for glass on B2O3 basis
Figure 7: Comparison of hydrated and anhydrous borates for glass on B2O3 basis
Note
[1] Glass Worldwide - Issue 92 Page 120-121 Nov 30, 2020
[2] Courtesy of CelSian
[3] T. Türkbay et al, Life Cycle Assessment of Boron Industry from Mining to Refined Products, Sustainability 2022, 14, 1787.
[4] J. An and X. Xue, Life cycle environmental impact assessment of borax and boric acid production in China, Journal of Cleaner Production, Volume 66, 1 March 2014, Pages 121-127.
Resources